题 目: Bimetallic Oxide Fe1.89Mo4.11O7 Electrocatalyst with Highly Efficient Hydrogen Evolution Reaction Activity in Alkaline and Acidic Media
作 者: Zhaomin Hao, Shishuai Yang, Jingyang Niu, Zhiqiang Fang, Liangliang Liu, Qingsong Dong,* Shuyan Song and Yong Zhao,*
刊 名: Chemical Science
年卷页: 2018, DOI: 10.1039/C8SC01710G
影响因子:8.668
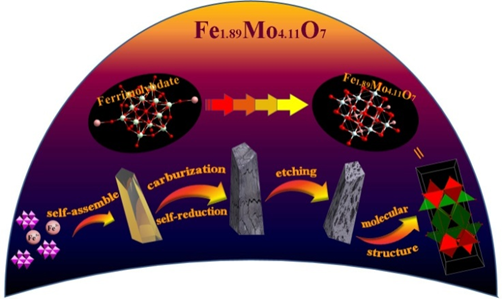
摘 要:Transition-metal Mo-based materials have been considered as one of the most effective hydrogen evolution reaction (HER) electrocatalysts. Regulating the electronic structure of Mo atom with guestmetal atom is considered as one of important strategies to improve their HER activity. However, intruduction of guest metal elements neighboring to Mo-site with atomic-level hybridization is difficult to be realized, resulting in the failure of modified electronic structure for Mo sites. Herein, a Fe1.89Mo4.11O7/MoO2 materialis prepared through the thermal treatment of ferrimolybdate precursor. It exhibits a Tafel slope of 79 mVdec-1 and an exchange current density of 0.069 mAcm-2 in 1 M KOH medium, as well as a Tafel slope of 47 mV dec-1 and an exchange current density of 0.072 mA cm-2 in 0.5 M H2SO4 medium. Compared to the original Mo-based oxides, Fe1.89Mo4.11O7 with the regulated Mo electronic structure shows the more suitable Mo-H bond strength for the fast kinetics of HER process. Density functional theory (DFT) calculation also indicates that the Mo-H bond strength in Fe1.89Mo4.11O7 is similar as that of Pt, achieving the high kinetic activity of Mo-based HER electrocatalysts in alkaline and acidic media.
文章链接:http://pubs.rsc.org/en/content/articlehtml/2018/sc/c8sc01710g.
